
Studies with Liv.52 in the Treatment of Infective Hepatitis, Chronic Active Hepatitis and Cirrhosis of the Liver
Prof. Mandal, J.N., M.B. (Cal.), D.C.H. (Lond.), F.R.C.P. (Edin.), Professor - Director
and Roy, B.K., M.D. (Cal.), Senior House Physician to Professor-Director, Department of Medicine, Medical College and Hospital, Calcutta, India.
Liv.52 in chronic active hepatitis and cirrhosis
Out of admitted cases in the Hospital only those cases who had not received any treatment beyond general measures were included in the study. After selection of the patients, all were clinically examined and the findings recorded in a proforma prepared for the purpose. The cases were then placed in the clinical categories indicated earlier.
Haematological and biochemical investigations were then carried out and recorded in respect of each patient. They were repeated every week to every month and every third month, as warranted by the category of cases. Haemogram included Hb, TC, DC, ESR, BT, CT and prothrombin time.
Blood biochemical studies included blood sugar, urea, Serum bilirubin, Total protein, Albumin/Globulin ration, Protein Electrophoresis, Alkaline phosphatase, SGOT and SGPT in each case. B.S.P. excretion test was done in 20 cases in the two groups of patients viz. Chronic Active Hepatitis and Cirrhosis, taking 5 Control and 5 Liv.52 cases from each group. HBsAg was tested in all cases. Skiagram of the chest, Barium-swallow of oesophagus and in some, Barium-meal X-ray of stomach and duodenum were done in cases of Cirrhosis.
Periodic liver biopsy was done in patients of each clinical category, excepting Infective Hepatitis.
Liver scientigram could be done only on six cases and had to be discontinued due to some unavoidable problems.
The patients were put on Liv.52 tab. (4 tablets, t.i.d. irrespective of age and sex) after thorough investigations, and the improvement in the patients were assessed clinically, haematologically, biochemically and histopathologically at periodic intervals. Haemogram and biochemical studies were repeated in each patient every month, and liver biopsy was repeated at intervals of 3 to 6 months.
After their discharge from the hospital, patients were directed to attend a special follow-up clinic of the trial at O.P.D. at periodic intervals, where they were reviewed and received the supply of drugs. They were readmitted from time to time for further investigations and assessment.
In both groups of cases (viz. Liv.52 Group and Control Group) patients received supportive treatments in the form of rest, nutritious diet, Vitamin B-complex, Glucose drinks and drip etc. Frusemide and spironolactone were used as and when indicated for the relief of oedema and/or ascites, but steroids were not used in any patient of either group.
The efficacy of the treatment was assessed by the improvement of subjective symptoms and objective signs, the degree of improvement in various Liver Function Tests, as well as the degree of histopathological improvement of liver.
OBSERVATIONS AND RESULTS
Group A: Infective Hepatitis: In the present study of 45 cases of Infective Hepatitis, 30 patients were put on Liv.52 and 15 cases served as Controls.
Symptoms in these cases included fever, anorexia, nausea, vomiting, right hypochondriac/epigastric pain, yellow coloration of urine and conjunctivae, prostration, boneache, headache, insomnia, flatulence and in some, clay-coloured stools.
All of them had slight to severe degree of jaundice and enlarged tender liver ranging from 1" and 2½" below right costal margin.
Clinical and biochemical improvements were observed in majority of cases earlier in Liv.52 Group than in Control Group.
1. Clinical: Symptomatic improvement was observed specially in respect of fever, anorexia, nausea, weakness, boneache, headache, insomnia, flatulence and epigastric pain earlier in Liv.52 Group than in Control Group. Disappearance of yellow coloration of urine and conjunctivae was also much earlier in Liv.52 Group than in Control Group. Similar improvement in physical signs like enlarged tender liver, jaundice etc. was observed in the Liv.52-treated cases.
2. Biochemical (Liver Function Tests)
| (i) Serum Bilirubin: The improvement in the level of Serum bilirubin in majority cases of Liv.52 Group was observed very early i.e. within 7 days’ of treatment and the value of Serum bilirubin became normal to near-normal within 2-3 weeks in majority of cases (73.33%) in Liv.52 Group, whereas the improvement in the level of Serum bilirubin in Control Group was observed much later i.e. after 2-3 weeks, in majority of cases. Serum bilirubin became normal only after 3-6 weeks in this group. The trend of changes in Serum bilirubin is depicted in Table IV and Annexure I. | 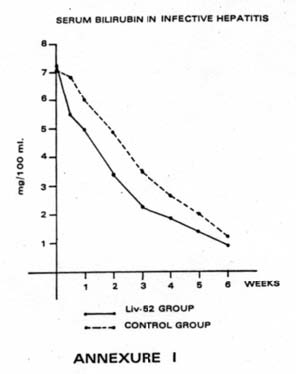 |
| Table IV: Serum bilirubin level in infective hepatitis (in mg) | ||||||||
| On admission | 3rd day | 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week | |
| Liv.52 group (Mean: 30 cases) | 7.23 | 6.49 | 4.98 | 3.31 | 2.24 | 1.85 | 1.34 | 0.98 |
| Control group (Mean: 15 cases) | 7.19 | 6.86 | 6.00 | 4.87 | 3.50 | 2.64 | 2.00 | 1.23 |
| (ii) Alkaline Phosphatase: Values of Alkaline phosphatase in the Liv.52 Group of cases showed earlier improvement in majority of cases (73.33%) than in Control Group (26.66%). Improvement was observed within 2-3 weeks in Liv.52 Group whereas in Control Group, majority of patients took 3-6 weeks to show improvement. The trend of changes in Alkaline phosphatase is depicted in Table V and Annexure II. | 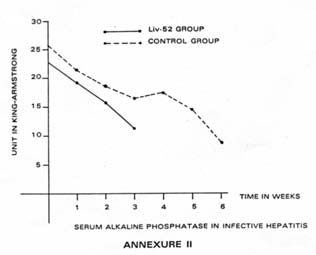 |
| Table V: Serum alkaline phosphatase in infective hepatitis (in King-Armstrong Units) | |||||||
| On admission | 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week | |
| Liv.52 group (Mean: 30 cases) | 22.97 | 19.20 | 15.8 | 11.14 | – | – | – |
| Control group (Mean: 15 cases) | 25.78 | 21.36 | 18.89 | 15.98 | 17.10 | 14.70 | 8.90 |

Copyrights © 2009 healthyliver.co.uk
